Product Development Co-op
I designed and tested surgical instruments during my co-op at Johnson & Johnson.
2023
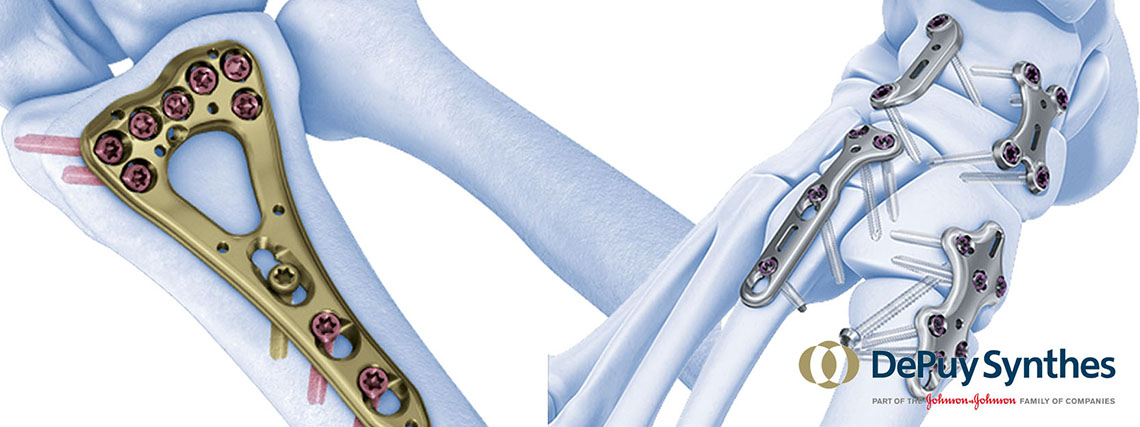
Overview
*Note: due to the nature of the work, some details are classified.
Design Work
researching the techniques, creating prototypes, and conducting tests in cadaver labs.
To ensure the success of the project, I engaged in multiple iterations and collaborated closely with a team of Senior Engineers, Global Marketing professionals, and Supply Chain experts. This collaborative effort was crucial in ensuring that my prototypes were not only
mechanically robust but also aligned with customer needs and feasible for manufacturing.
This big of a project presented an opportunity for significant personal growth and learning. Here are some key aspects I delved into:
•
Universal Application:
I ensured that the instruments I designed would be universally applicable by utilizing digital analysis on thousands of bone models.•
Design for Manufacturability:
Collaborating with fellow team members, I created complex designs that were not only innovative, but also manufacturable.•
Strength Assurance:
Employing both physical testing and digital analysis on ANSYS softwsare, I meticulously verified that the instruments would retain their strength even after hundreds of uses.•
Material Selection:
Recognizing the need for enhanced efficiency in material selection, I compiled and presented an extensive catalog encompassing a wide array of 3D printed and machined materials to hundreds of employees wordwide.The culmination of these efforts was marked by engagements with surgeons. This offered a firsthand experience of the impact my designs had on their practice, and was very encouraging when they were met with
positive feedback!
This project encapsulated a compelling narrative of innovation, collaboration, and tangible impact.
It underscores the power of enginering to bridge gaps, elevate medical practices, and contribute to the realm of surgical precision.
Mechanical Testing
My responsibilities encompassed producing comprehensive and meticulously regulated test reports, performing statistical analyses on outcomes, and overseeing the design and rapid prototyping of more than 20 test fixtures. The following are a few projects:
•
Tolerance Analysis:
Ensured that parts under both least material condition and most material condition adhered to safety requirements.•
Mechanical Strength:
Conducted detailed analyses and comprehensive reports on project components, comparing them to existing products to verify their superior strength.•
Test Protocol Development:
Assisted in creating comprehensive test protocols and procedures, including fixture design and instrumentation setup, to ensure accurate and reliable mechanical testing of medical devices.With an unwavering commitment to patient safety and a strong dedication to pushing the boundaries of innovation in the market, I contributed to a reduction in Time to Market, ultimately
benefiting both patients and the industry as a whole.

Independent Ventures
President of the Intern and Co-Op Association.
My responsibilities encompassed overseeing the onboarding and training of new hires, as well as organizing events. This ranged from conducting insightful tours of different facilities to arranging visits to other Johnson & Johnson operating companies, thereby providing us with a comprehensive understanding of the company and a multifaceted glimpse into the realm of engineering.Furthermore, fueled by my interest for computer programming, I sought active engagement in the
development and oversight of an internal website utilized by thousands of employees across the world.
I assumed responsibility for swiftly implementing requested enhancements, diligently upholding maintenance, and rigorously conducting bug testing.